The Reactants Needed to Produce Simple Polyesters Are
Place a few tablespoons of calcium hydroxide Ca OH 2 in a clear 500 ml reagent bottle and fill with water. You wouldnt expect your polyester fleece to fall to pieces if you went out in the rain.
Chapter 17 Flashcards Easy Notecards
Polyethylene terephthalate the polyester with the greatest market share is a synthetic polymer made of purified terephthalic acid PTA or its dimethyl ester dimethyl terephthalate DMT and monoethylene glycol MEG.
. B diacids and diamines. Before going direct into the main topic What type of chemical reaction produces a polymer let us understand the basics of polymer first. D alkenes and catalysts.
Ethylene the common monomer for these polymers is a low boiling -104º C gas. They can be formed from the reaction of a diacid or acid anhydride and a diol with the elimination of water or by ring-opening polymerization of cyclic di-esters. Shake or stir to make a cloudy suspension.
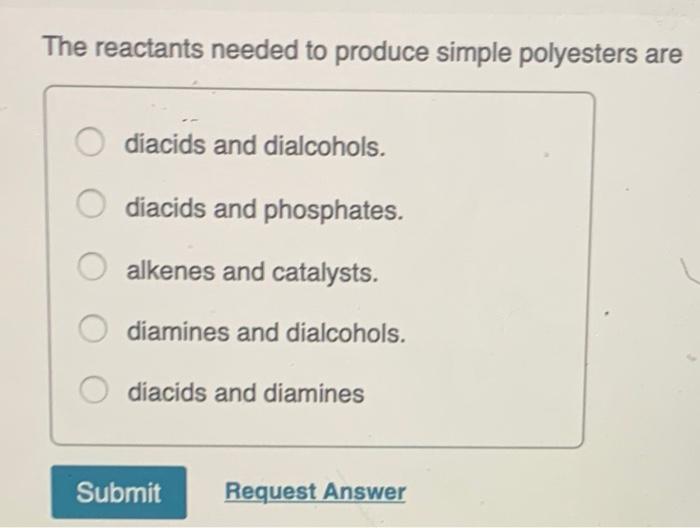
Simple amides are easily hydrolysed by reaction with dilute acids or alkalis. These two functional groups react to form the characteristic ester linkage a chemical group with the structure. 53 The reactants needed to produce simple polyesters are.
Leave the suspension to settle for a few days. Simple esters are easily hydrolysed by reaction with dilute acids or alkalis. A polyester forms from two different monomers.
Polyesters most commonly are prepared from a condensation reaction between an organic alcohol containing hydroxyl OH groups and a carboxylic acid containing carboxyl COOH groups. Ethyene glycol and the acid set including acids such as phthalic acid or maleic acid. Enone of these 49 50The products of acid hydrolysis of an ester are.
What is a Polymer. Diacids and diamines Submit Request Answer. Its helpful to understand some of the chemistry behind the polymerization reaction to appreciate how the process works and the complexity involved in making plastic.
Hydrolysis is faster at higher temperatures. The reactants needed to produce simple polyesters are diacids and dialcohols. In 1953 an inexpensive process for the separation of the various xylene isomers by crystallization.
Although aromatic polyesters had been successfully synthesized from the reaction of ethylene glycol with various aromatic diacids almost always terephthalic acid or its ester commercialization of polyester synthesis awaited an inexpensive source of aromatic diacids. Polyamides are fairly readily attacked by strong acids but are much more resistant to alkaline hydrolysis. Polyesters are one of the most important classes of thermoplastic polymers.
Up to 24 cash back 49One requirement for the reactants in the formation of polyester is that each molecule contain Aan amine group somewhere on the carbon skeleton. Ethylene C 2 H 4 is a stable molecule with two carbon atoms and a double bond. Dat least two functional groups that can form ester linkages.
In 1939 Whinfield and Dickson used an aromatic carboxylic acid terephthalic acid in combination with ethylene glycol to produce the first commercially viable polyester polyethylene terephthalate PET. C diamines and dialcohols. Polyesters are attacked readily by alkalis but much more slowly by dilute acids.
Natural latex rubber is an opaque soft easily deformable solid that becomes sticky when heated above. A polymerization reaction starts with a primary ingredient monomer such as ethylene or propylene. A diol which contains two alcohol groups OH.
R and R represent the linked. The clear liquid above the solid Ca OH 2 is a saturated solution of Ca OH 2 also known as clear limewater. Esterification is a process or a general name for a chemical reaction in which two reactants alcohol and an acid form an ester as the reaction product.
They consist of low molecular weight liquids used as plasticizers and as reactants in forming urethane polymers and linear high molecular weight thermoplastics such as polyethylene terephthalate Dacron and Mylar. The first part of the name n-pentyl refers to the alcohol needed to make the ester in this case n-pentyl alcohol 1-pentanol. For example ethane diol HO-CH 2 -CH 2 -OH can.
E diacids and phosphates. Hydrolysis by water alone is so slow as to be completely unimportant. A simple polyester can be made from a monomer which has two hydroxyl groups -OH and another monomer which has two carboxylic acid groups -COOH.
60º C and brittle when cooled below -50º C. For the creation of polyester resins two sets of reactants are chiefly used namely polyols which include glycols eg. Hydrolysis by water alone is.
A diacids and dialcohols. The formula for carboxylic acid esters is RCOOR where R and R are any organic combining groups that are prepared. Next use structural formulas to write out the.
Both LDPE and HDPE become brittle at very low temperatures below -80º C. Usual reactants for the saturated polyesters are a glycol and an acid or anhydride. This is another term that we need to learn and understand this topic.
According to the composition of their main chain polyesters are classified as aliphatic semi-aromatic and aromatic see table below. The byproduct water is steadily removed till the completion of the reactions. A dicarboxylic acid which contains two carboxylic acid groups COOH.
The second part of the name hexanoate refers to the carboxylic acid needed in this case hexanoic acid drop -ate and add -ic acid. Cat least one carbon-carbon double bond.
Solved The Reactants Needed To Produce Simple Polyesters Are Chegg Com
Comments
Post a Comment